Hitachi Constructs Japan’s First Platform for Integrated Management of Cell and Tracing Information Throughout the Value Chain for Regenerative Medicine Products Through Collaborative Creation With Alfresa and Others
Practical use as common service infrastructure for regenerative medicine products to begin from 2021
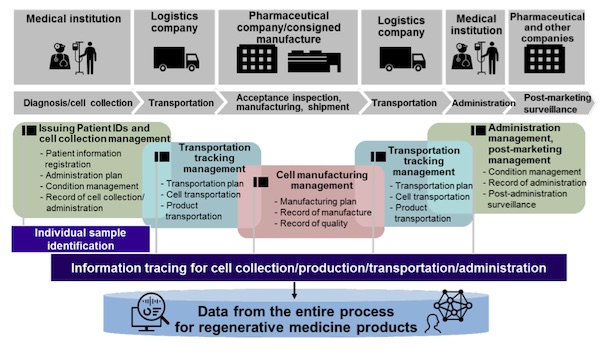
Hitachi, Ltd. has constructed a platform for the integrated management of cell and tracing information throughout the value chain, from cell collection, through production and transport, to the administration of regenerative medicine products(1) through collaborative creation with Alfresa Corporation (Alfresa), pharmaceutical companies, medical institutions, and others. Hitachi will start the practical operation of the platform in 2021 following an operational test and Alfresa will be one of the first users of the platform. The platform will individually identify and trace samples, throughout the processes of cell collection, transportation, and administration of regenerative medicine products that require strict quality control and information traceability. It is the first common service infrastructure(2) for regenerative medicine products in Japan that involves all stakeholders throughout the value chain (medical institutions, pharmaceutical/logistics/
Hitachi will use this platform to support the healthcare and pharmaceutical industries as one of its Lumada solutions for accelerating digital innovation, explore potential applications for specialty pharmaceuticals(3) that require rigorous information management in the distribution and aim for overseas deployment. Moreover, Hitachi will contribute to improving the social, environmental and economic value of its global customers in these industries and a better Quality of Life for everyone.
Recommended AI News: Deepgram Pioneers Novel Training Approach Setting New Standard for AI Companies
Alfresa distributes products such as ethical pharmaceuticals, medical devices/materials, and diagnostic reagents to medical institutions including hospitals, clinics, and dispensing pharmacies while fully controlling quality and ensuring reliability and safety. The company owns 13 logistics/pharmaceutical centers and is one of the key operating companies of the Alfresa Group, which is Japan’s top wholesaler of ethical pharmaceuticals(4). Making use of a substantial expertise and know-how that have been cultivated through the distribution of pharmaceutical products, Alfresa has established a high-quality distribution system in a specialty pharmaceutical field requiring strict temperature control and the traceability of each product.
In many countries, research and development is on-going on regenerative medical products that are expected to open up paths to new therapies for diseases for which there have been no effective treatment methods. A characteristic of regenerative medical products is that cells used in administration are collected from the patient him/herself or cell donor, incubated and injected to the patient. Thus, it is necessary to manage individual cells and data for tracing them in every process throughout the supply chain. In this context, a brand-new supply chain system is desired for the reliable and safe distribution of regenerative medical products. Therefore, it is becoming more important that the infrastructure can collect, store and use data from diverse stakeholders by utilizing the state-of-the-arts digital technologies including IoT and AI.
Hitachi Group boasts a substantial track record and knowledge, having provided production equipment including culture equipment, control/IT systems including production/quality control systems, Enterprise Resource Planning (ERP), and electronic medical chart systems for years. Hitachi also has a wide lineup for regenerative medicine field, which is expected to spread, including cell culture apparatuses, safety cabinets, and cell processing/preparation equipment. Utilizing its technology and knowhow, including these products, operational technology (OT) and information technology (IT), Hitachi has developed and constructed this platform to improve and optimize the entire regenerative medicine product value chain from cell collection, production and transportation to administration.
Recommended AI News: Auraria Campus Partners With ParkMobile to Offer Contactless Payments on Denver Campus
In regenerative medicine, Alfresa has arranged storage and transportation environment realizing an ultra-low-temperature, which is -150 degC or lower, utilizing liquid nitrogen equipment and others through its Tonomachi Regenerative Medicine Distribution Station in Kawasaki, Kanagawa. In addition, Alfresa is accumulating expertise and know-how on storage management and transportation of clinical testing products and regenerative medical products through capital and business alliances with other companies specialized in regenerative medical products. Alfresa will successfully unify management and improve the efficiency of distribution by participating in the early stages of the platform’s development and sharing Hitachi with Alfresa’s dedicated knowledge regarding the transportation materials and transportation arrangements. The platform will enable Alfresa to improve its reliable and safe supply system for regenerative medicine products to medical institutions and patients.
Outline and Features of the platform
The platform will be common infrastructure for regenerative medicine products for services that can be used by stakeholders at the medical institutions and pharmaceutical/logistics/
1. Information integrated through the combination of experience in system construction and solutions of Hitachi
– Individual identification: Hitachi utilizes its experience and knowhow to link patient IDs and operation instruction data for projects related to regenerative medicine at universities and medical institutions, and are reflected in the system.
– Information tracing: Hitachi’s digital twin solution has been employed as a part of information infrastructure to trace quality information in supply chains, including cell collection, production, transportation and administration. The digital twin solution has a proven record, having already been utilized in the manufacturing industry.
2. High practicality secured through collaborative creation with stakeholders Alfresa’s knowledge was used to develop the transportation and storage of patient cells and regenerative medicine products. Knowledge from consigned manufacturers was used to develop manufacturing methods and manufacture experimental drugs and regenerative medicine products. Knowledge of pharmaceutical companies and bio- venture companies was leveraged to ensure the traceability of the whole process and information from the collection of cells from patients to the administration of regenerative medicine products. The resulting specifications focus on the standard in use by a wide range of stakeholders, taking into account practical concerns. Moreover, to achieve their efficient use in medical institutions, opinions from healthcare professionals are incorporated in the development of user interfaces.
3. Scheduling and order receiving/placing management covering the entire process It is important to manage the schedule modifications caused by many reasons throughout the process from therapy/manufacturing planning and cell collection to the administration of regenerative medicine products. While sharing the situation of each stakeholder, it enables to collectively plan and adjust optimal schedule as well as manage sales orders covering the entire value chain. In the future, more features will be added, such as automated scheduling based on plan histories.
4. Facilitation of analysis and simulation overlooking the entire supply chain
Hitachi’s digital twin solution, which was employed as the information infrastructure of the system, facilitates timely, continuous AI-based analysis and simulation and optimizes the entire production process by linking OT data including equipment operations and quality information with IT data including plan and inventory management in a digital space. This will help improve efficiency and productivity based on analysis and simulation covering the entire process from cell collection to administration. This feature will be introduced according to customers’ needs.
(1) Products that have been processed under the responsibility of a corporation among cell processed products used for cell therapy/gene treatment/regenerative medicine
(2) Surveyed by Hitachi, as of August 31, 2020
(3) Pharmaceutical products for rare diseases and those for regenerative medicine
(4) Source: 2020 ALL DATA & RANKING issued by Drugmagazine, Co., Ltd.
Recommended AI News: Nous Infosystems Transitions to ServiceNow’s Premier Partner Program Segment

Comments are closed, but trackbacks and pingbacks are open.